Internally Reversible Process
A process is called internally reversible if no irreversibilities occur within the boundaries of the system during the process. During an internally reversible process, a system proceeds through a series of equilibrium states, and when the process is reversed, the system passes through exactly the same equilibrium states while returning to its initial state. That is, the paths of the forward and reverse processes coincide for an internally reversible process. The quasi-equilibrium process is an example of an internally reversible process.
For an internally reversible process, the entropy balance in a closed system equation becomes
since there is no entropy generation .
The above equation can be re-arranged to become
such that we can quantify the amount of heat transfer in the system due to change in entropy at a given temperature.
P-V : T-S Analogy - Derivation of TdS Equations
Like how moving boundary work can be expressed as , where the area under the P-V curve is the work output, we have:
where the area under the Temperature-Entropy (T-S) curve is the heat input.
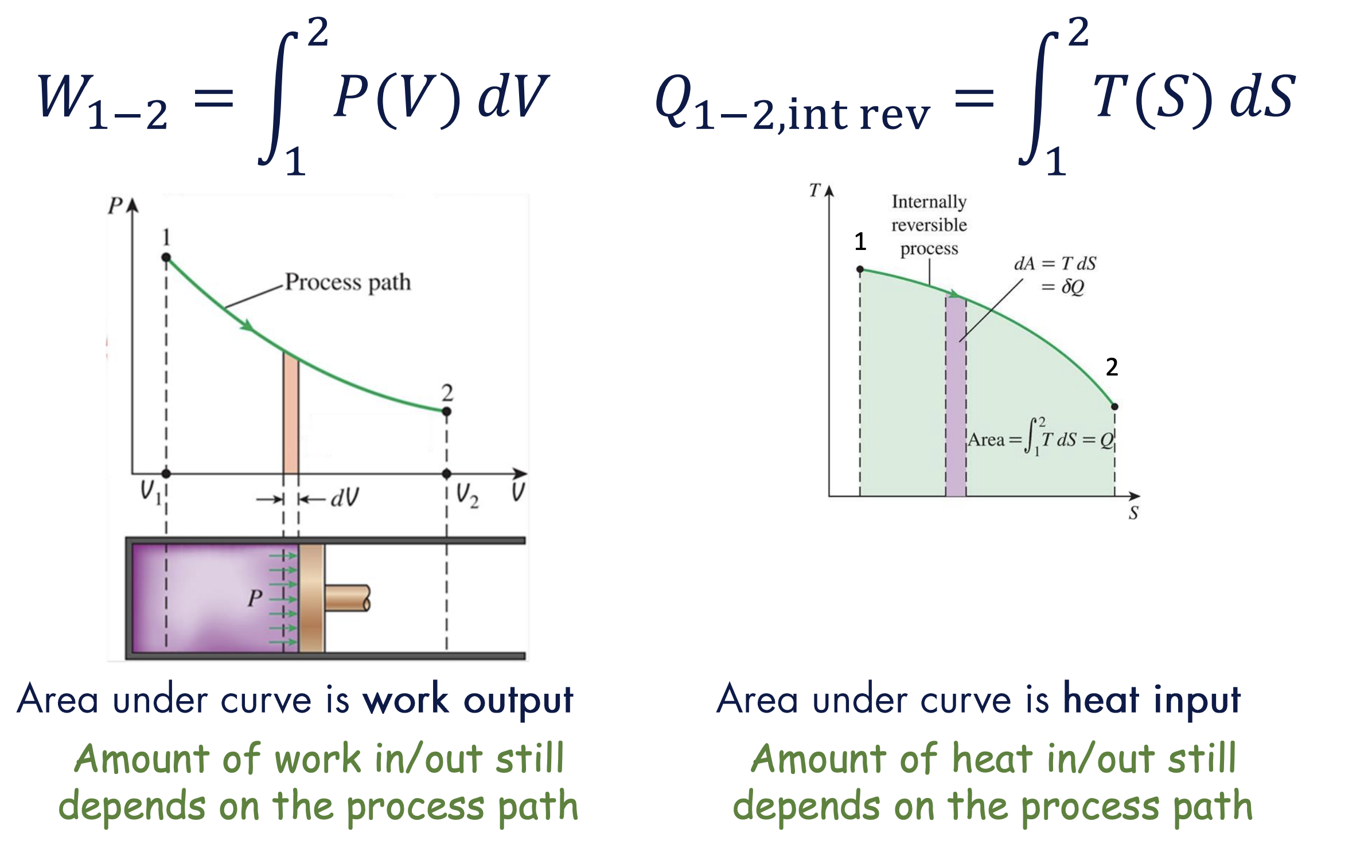
For boundary work, we can write:
Similarly, for internally reversible heat transfer, we can write
Recall that the first law states
So we have:
or
This is a nice formula because can be found in data booklet as intensive independent properties.
The TdS Equations
Recall that we can write enthalpy as
In differential form:
Since we established that , we can then write:
Thus, considering a stationary, closed system undergoing a reversible process between State 1 and State 2, we arrive at the TdS equations:
- The TdS equations relate state properties. Thus, in order to use them, equation of state must be defined, such as the P-v-T surface. See Entropy Change of Pure Substances.
- The TdS equations relate state properties, so they do not rely on the process path. They are valid for reversible or irreversible processes and closed/open systems. This is a bit of a bait and switch since we specifically used internally reversible processes to do this derivation.
- To use the TdS equations, and during the process must be known, and entropy may still be generated.
Applying TdS Equations
Recall that we apply specific heat, , differently for each phase of matter to relate or to .
- For solids or incompressible liquids, we can say that .
- In or near the vapor dome, we use tabulated for
- For ideal gases, we use and .
Similarly, we can apply the TdS equations in convenient forms depending on the phase.
Solids + Incompressible Liquids
For solids or incompressible liquids, since the volume cannot change, we have . We can also write change in internal energy in terms of change in temperature as . Then, our TdS equations, we have:
We can then write:
Note that in the isentropic case, we have and .
Vapor Dome
In or near the vapor dome, we use the property tables with to find .
Ideal Gases
For ideal gases, we have and , which lets us write
This leads to
Example
Example 1
Example 2