In a closed system, the entropy balance can be given as:
where:
- is the change in total entropy of the system
- is the total entropy entering - total entropy leaving
- is the total entropy generated
The above can also be written in differential form:
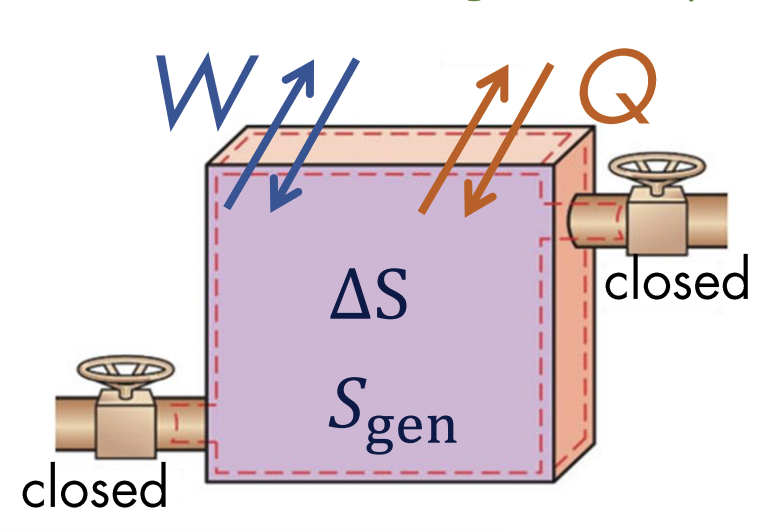
We can also write:
where is the sum of all differential amounts of heat transfer divided by temperature at location on the boundary. We can interpret this equation as:
- is the change in number of microstates
- is the change in number of microstates due to heat addition
- is the change in number of microstates due to irreversibilities
Recall that we always have:
Some more things to note:
- Adding/subtracting work does not change the number of microstates (does not change entropy).
- Adding heat results in a large increase in number of microstates if the current temperature is low, or a small increase in number of microstates if the current temperature is already high.