Specific heat is the amount of energy required to raise the temperature of a unit mass of a substance by . This is defined as:
and we also have
The above assumes that internal energy is only a function of temperature, such as an ideal gas.
Specific heat is not fixed; it changes as a function of temperature.
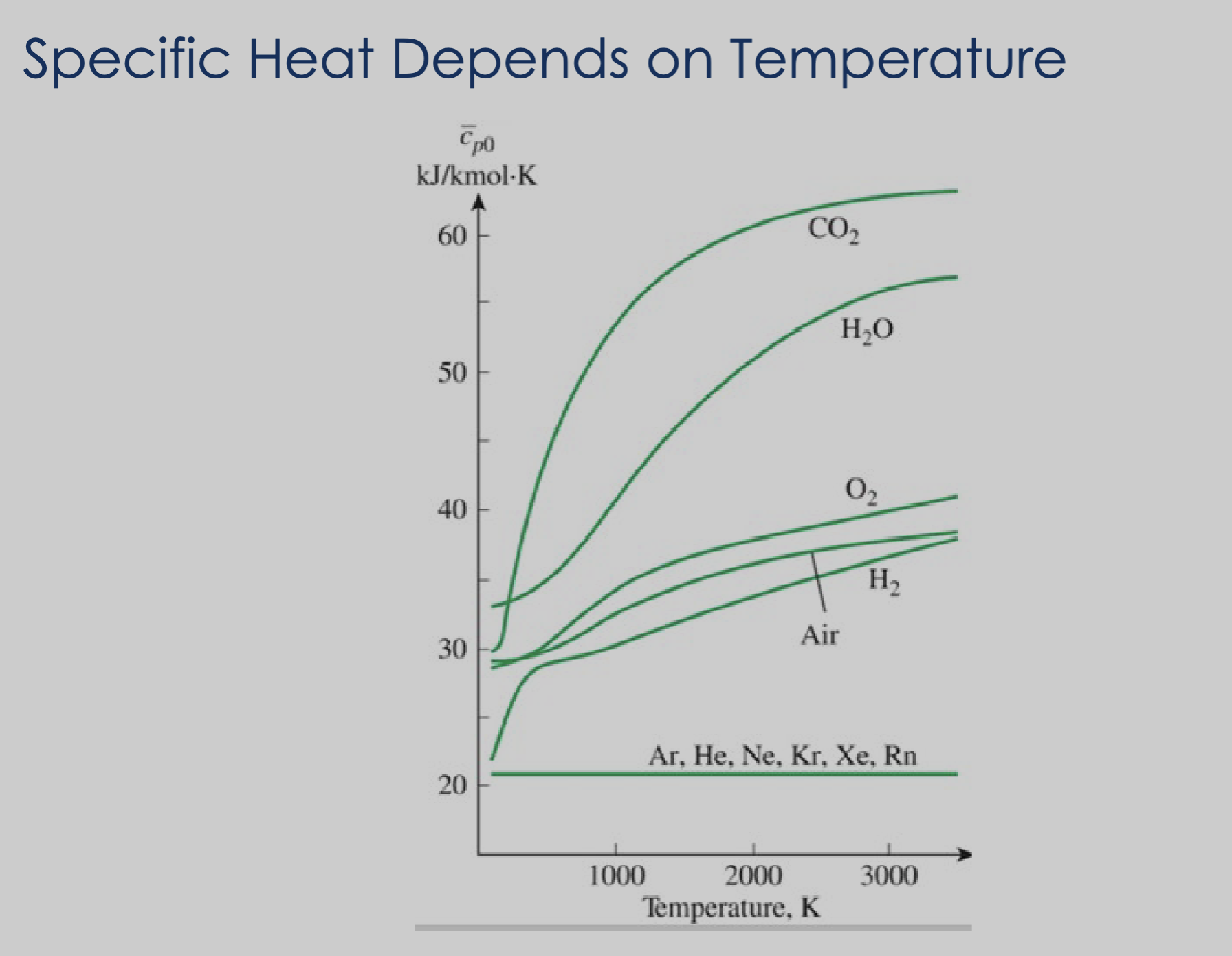
See: