Like how pure substances have T-v diagrams, we can also draw T-s diagrams plotting entropy against temperature. Entropy just provides another independent intensive property.
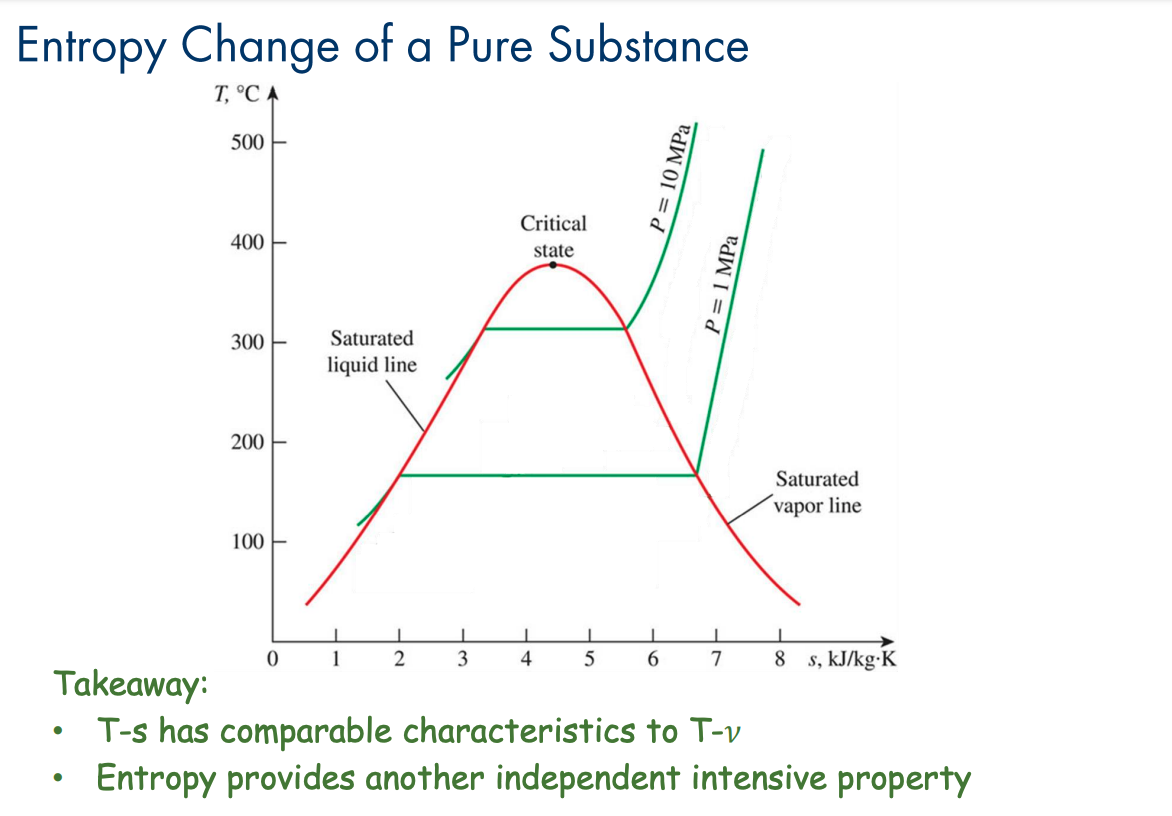
These are essentially the plotted version of the TdS Equations.
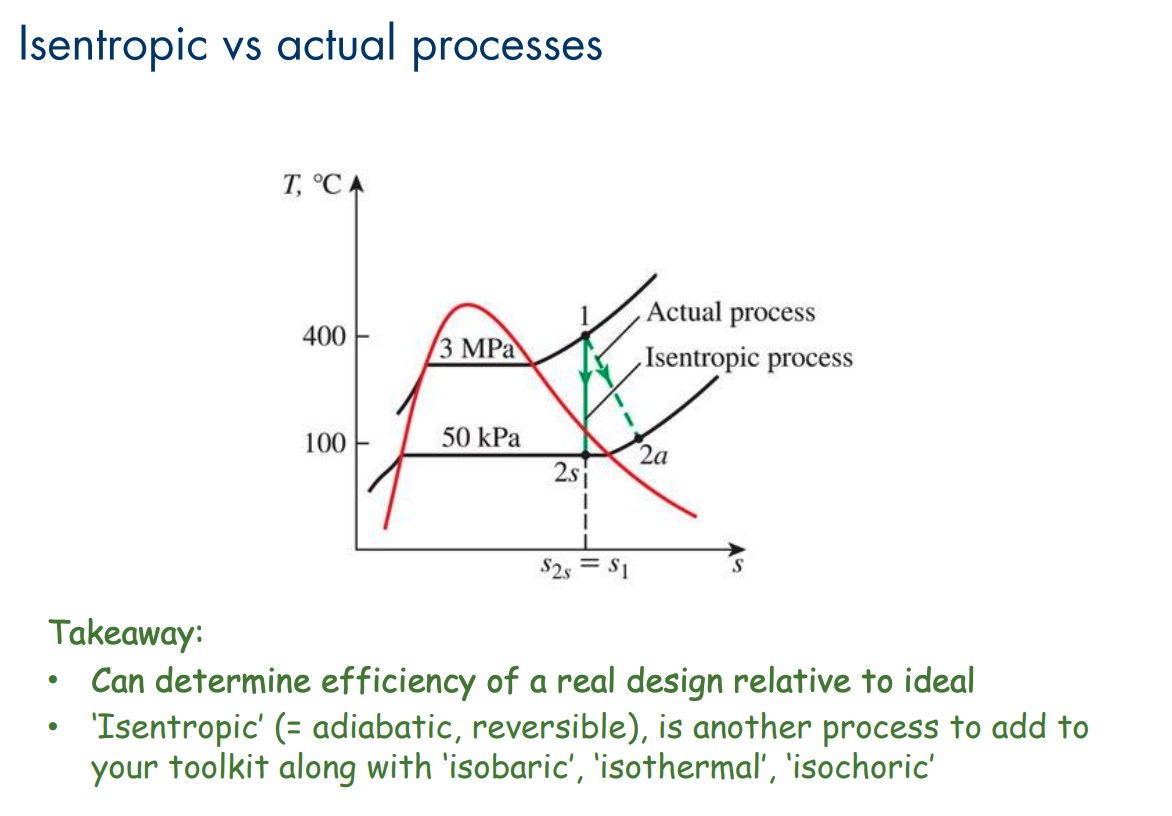
An isentropic ideal process would move directly vertically on the T-s plot. We can determine the efficiency of a real design relative to the ideal by comparing the diagrams.