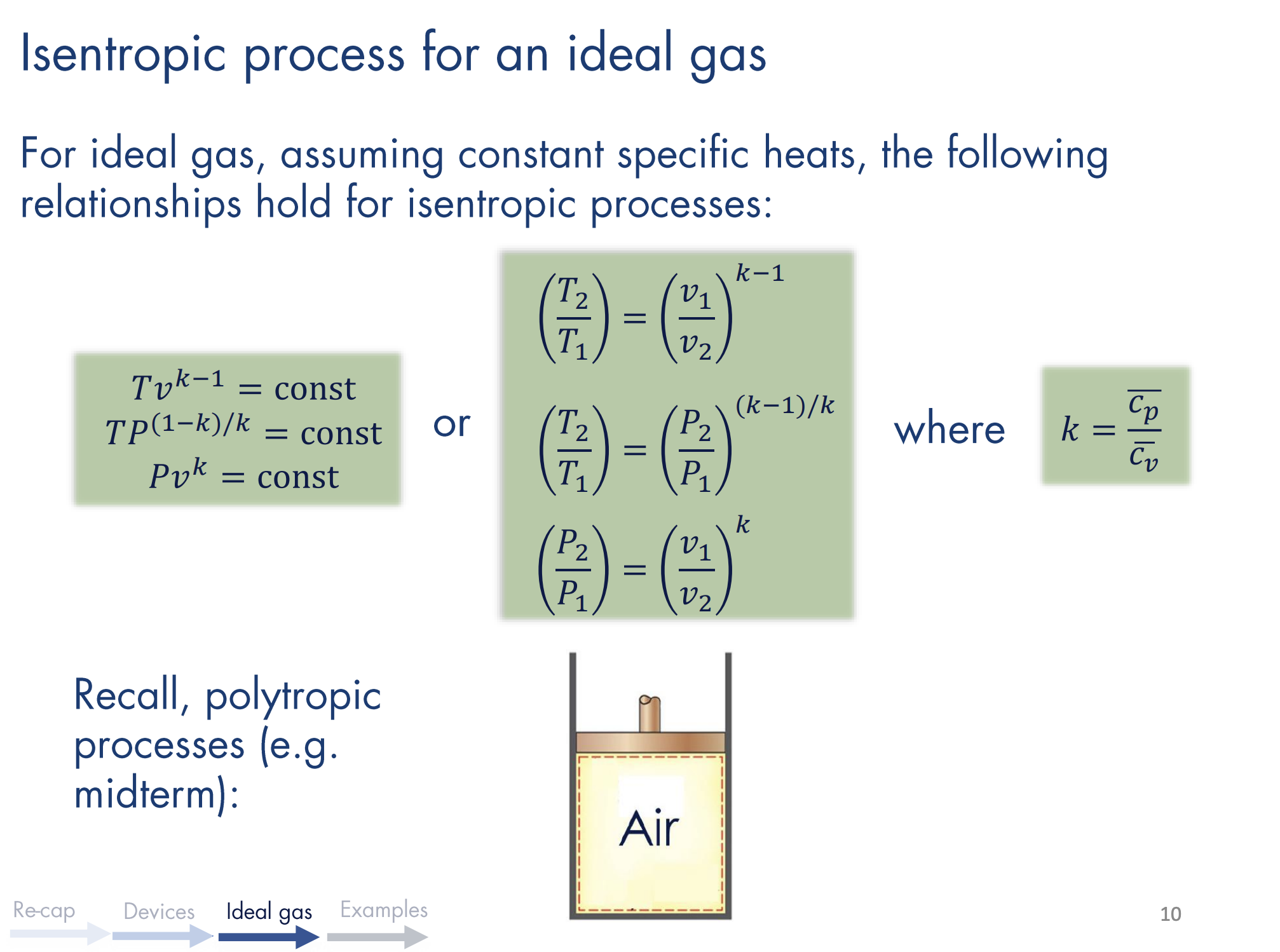
Recall that the TdS Equations state that for ideal gases, we have:
For an isentropic process, , so that . This means
Recall that the specific heat ratio is given as , and . Thus, we have:
So:
By the ideal gas equation, we also have:
which is in the form of a polytropic process. Thus, the polytropic process is isentropic (reversible and adiabatic) if , where .