In a heat engine, we have
represents the magnitude of the energy wasted in order to complete the cycle. But is never zero; thus the net work output of a heat engine is always less than the amount of heat input. Only part of the heat transferred to the heat engine is converted to work.
The fraction of the heat input that is converted to net work output is called the thermal efficiency . For heat engines, the desired output is the net work output, and the required input is the amount of heat supplied to the working fluid. Then, the thermal efficiency is:
Cyclic devices such as heat engines, refrigerators, and heat pumps, operate between a high temperature medium (or reservoir) at temperature and a low temperature reservoir at temperature . For uniformality, we define these quantities:
- is the magnitude of heat transfer between the cyclic device and the high-temperature medium at temperature .
- is the magnitude of heat transfer between the cyclic device and the low-temperature medium at temperature .
Note that and are defined as magnitudes, and are therefore positive quantities. Their direction can be determined by inspection.
Then, the net work output and thermal efficiency relations for any heat engine can be expressed as:
Thermal efficiency is a measure of how efficiently a heat engine converts the heat that it receives to work. Heat engines are built for the purpose of converting heat to work, and engineers are constantly trying to improve the efficiencies of these devices. The Kelvin-Planck statement of the 2nd Law says that .
Thermodynamic Temperature
So we know that a heat engine like a steam power plant operates in a cycle to continuously use heat to generator work. For the cycle, we can write:
and
Here, is defined as a thermodynamic temperature scale by Lord Kelvin. Energy is 0 at 0 Kelvin.
Carnot Efficiency
The maximum thermal efficiency is called the Carnot efficiency:
or:
- As , is as useful as .
- As , is useless (no driving force).
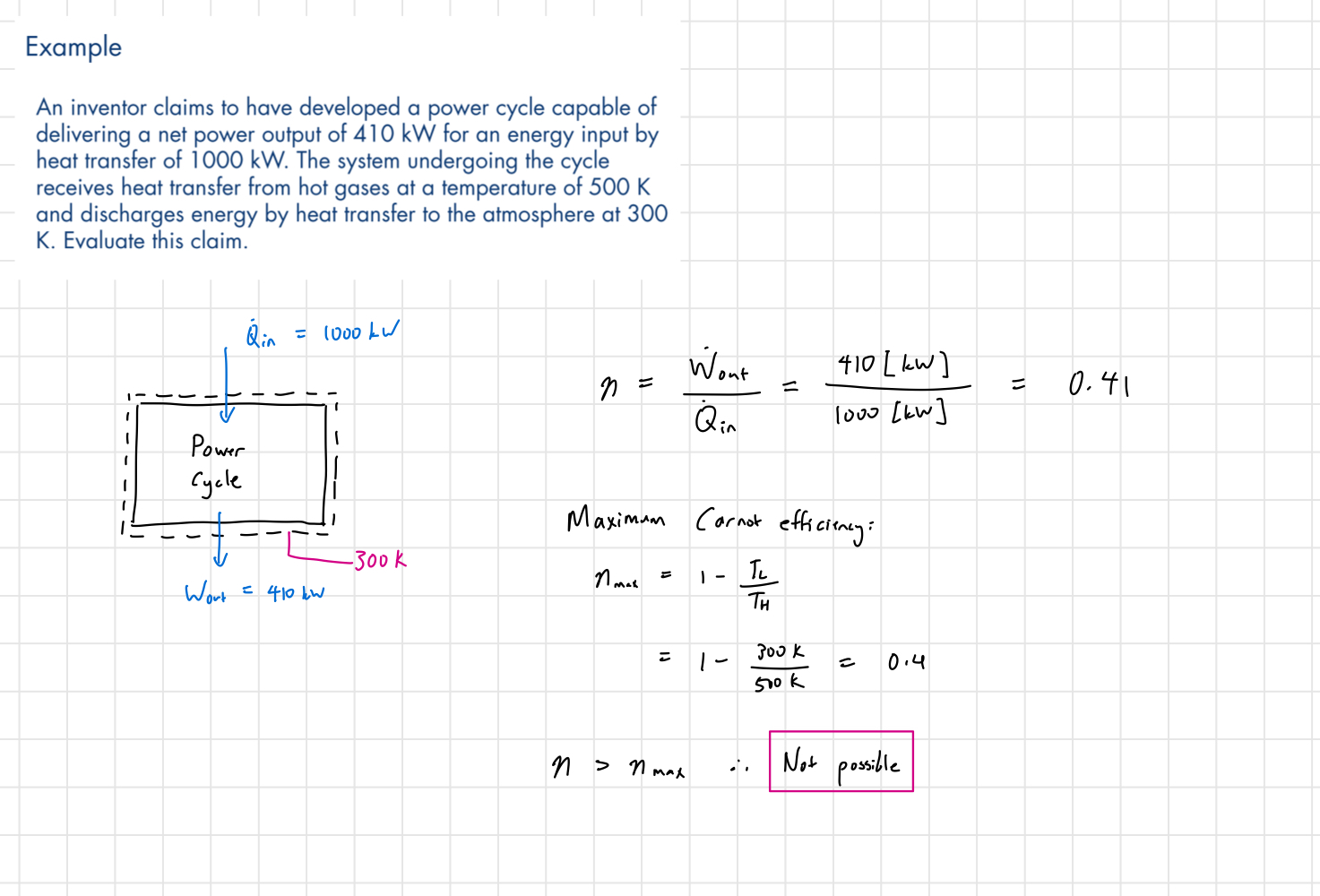
Example