Mechanical work done by a shaft submerged in water to the internal energy of the water; this energy may then leave the water as heat. However, any attempt to reverse the process will fail. ==Work can be converted to heat directly and completely, but converting heat to work requires the use of special devices called heat engines.==
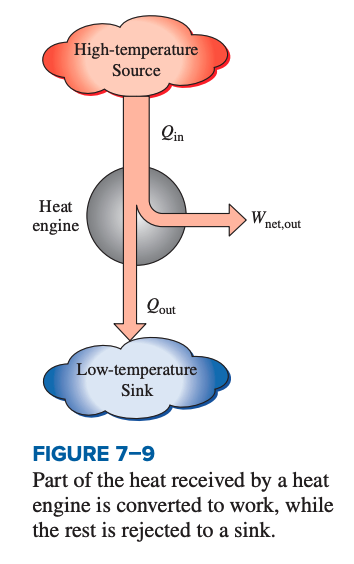
Heat engines all follow these principles:
- They receive heat from a high-temperature source (solar energy, oil furnace, nuclear reactor, etc.)
- They convert part of this heat to work (usually in the form of a rotating shaft)
- They reject the remaining waste heat to a low-temperature sink (the atmosphere, rivers, etc.)
- They operate on a cycle.
Heat engines and other cyclic devices usually involve a fluid to and form which heat is transferred while undergoing a cycle. This is called the working fluid.
Steam Power Plant
A steam power plant is an example of a heat engine. Combustion takes place outside the engine, and the thermal energy released during this process is transferred to the steam as heat. The schematic of a basic power plant is shown.
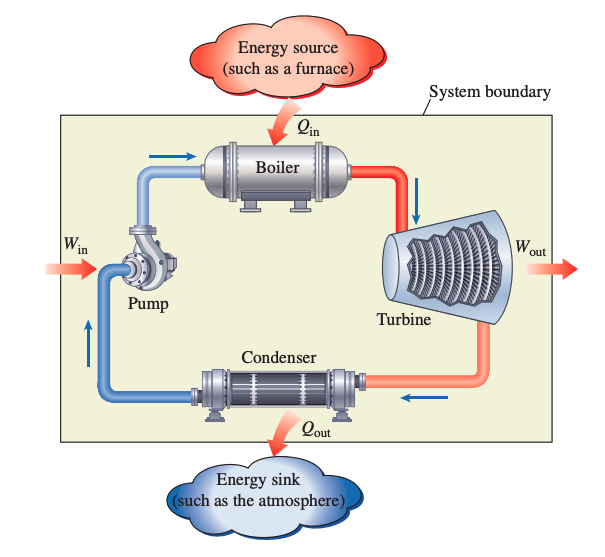
- is the amount of heat supplied to the steam in boiler from a high-temperature source.
- is the amount of heat rejected from steam in condenser to a low-temperature sink
- is the amount of work delivered by steam as it expands in turbine.
- is the amount of work required to compress water to boiler pressure.
The net work output is then:
Recall that for a closed system undergoing a cycle, the change in internal energy is zero, and therefore the net work output of the system is also equal to the net heat transfer to the system: