Previously we applied the first law of thermodynamics, or the conservation of energy principle, to processes involving closed and open systems. This states that energy is a conserved property. However, satisfying the first law alone does not ensure that a given process will take place.
For example, hot coffee getting even hotter in a cooler room as a result of heat transfer from the room air does not violate the first law but we know that it never takes place. We see that processes proceed in a certain direction and not in the reverse direction. Such violations of the second law can be detected by using the property of entropy.
The second law also asserts that energy has quality as well as quantity. The first law is concerned with the quantity of energy and the transformations of energy from one form to another with no regard to its quality.
For a process to proceed, both the first and second laws must be satisfied (direction).
Reversibility
Irreversibilities
All physical processes contain irreversibilities that transform energy from an organized (useful) state to a disorganized (less useful) state.
- Examples of irreversibilities:
- Friction/drag
- Heat transfer due to a finite temperature difference
- Fast or unconstrained expansion/compression
- Mixing of different species
- Inelastic deformation
- Chemical reactions
Reversible Processes
The system could possibly be restored to its initial state (“internally reversible”), but would then not be possible to also return the surroundings to their initial state.
Reversible processes are conceptual, idealized processes where the system and surroundings can be exactly restored to their initial states after the process has taken place.
- Examples of reversible processes:
- Frictionless motion
- Quasi-equilibrium expansion/compression
- Elastic deformation
These are used to determine the best theoretical performance of cycles, engines, and other devices. We can then quantify factors which reduce performance from maximum.
Statements of the Second Law
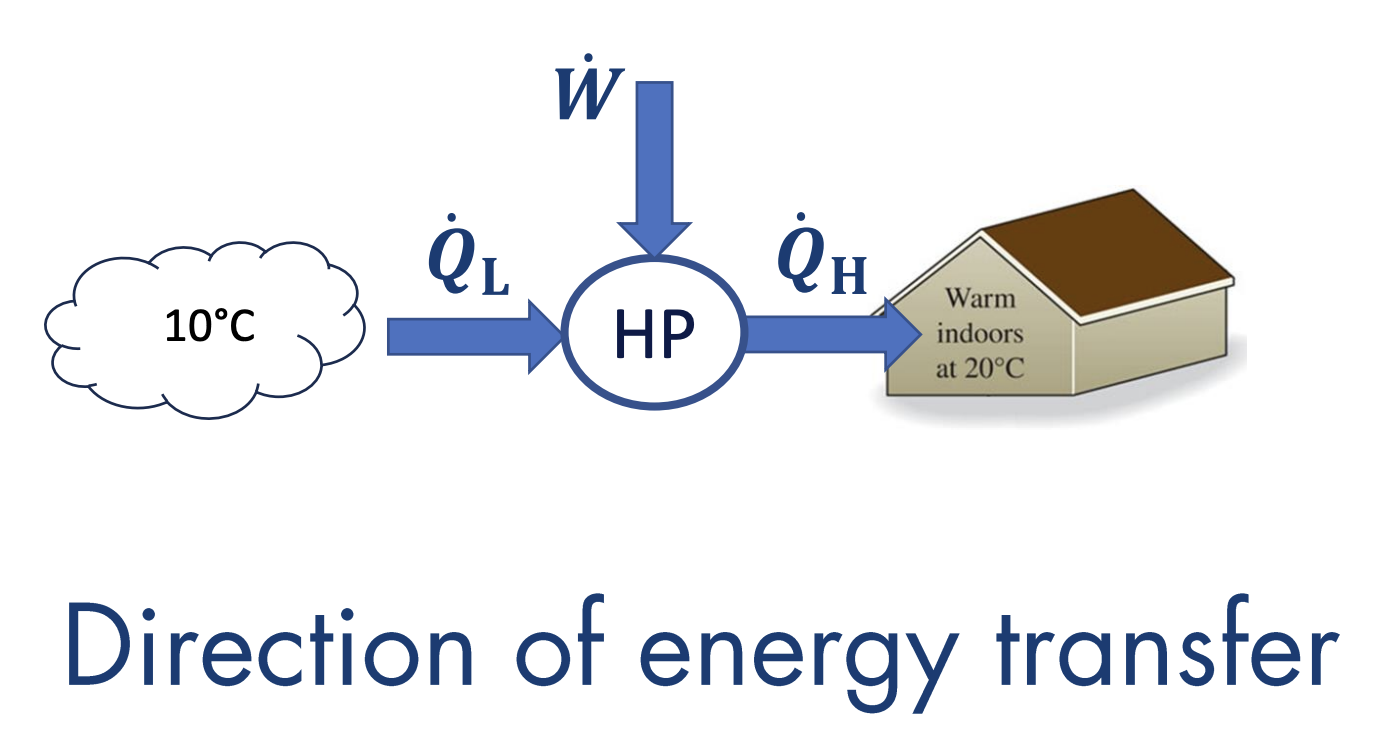
Clausius Statement of the Second Law
A system cannot operate so that its sole outcome is heat transfer from a cold reservoir to a hot reservoir.
This statement deals with the direction of energy transfer.
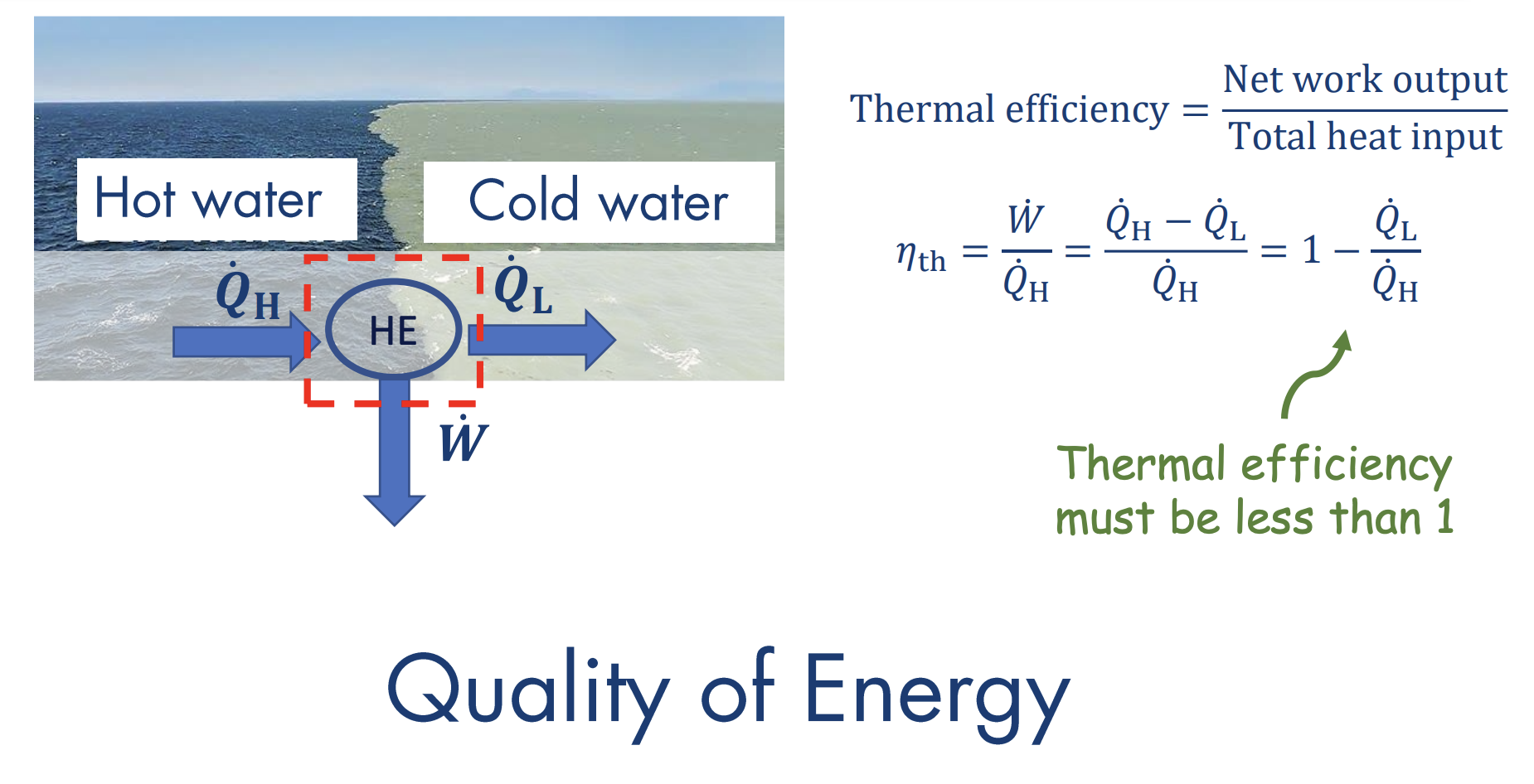
Kelvin-Planck Statement of the Second Law
No system can transform all heat into work. There must always be some heat rejection.
This deals with thermal efficiency, where:
This statement basically says that: