Phases:
- Subcooled liquid – Liquid is at a temperature below its boiling point at a given pressure. The liquid is not about to vaporize (fully liquid). Left of point on phase diagram.
- Saturated liquid – Liquid is at its boiling point and is about to start vaporizing. At this point, any addition of heat will start converting the liquid into vapor without increasing the temperature. Point of on phase diagram.
- Two-phase mixture – Consists of both liquid and vapor phases in equilibrium. In this region, the temperature and pressure remain constant during the phase change. The quality (dryness fraction) defines the proportion of vapor in the mixture.
- Saturated vapor – A saturated vapor is a state where the vapor is at its condensation point and is about to start condensing. At this point, any removal of heat will start converting the vapor into liquid without decreasing the temperature. Point on the T-v diagram.
- Superheated vapor – A superheated vapor is a state where the vapor is at a temperature above its condensation point at a given pressure. In this state, the vapor is not about to condense. It is fully in the vapor phase and is above the temperature required for condensation at the current pressure. Right of point on the T-v diagram.
- Critical point – Distinction between liquid and vapor phases ceases to exist.
How do we identify the state of a substance?
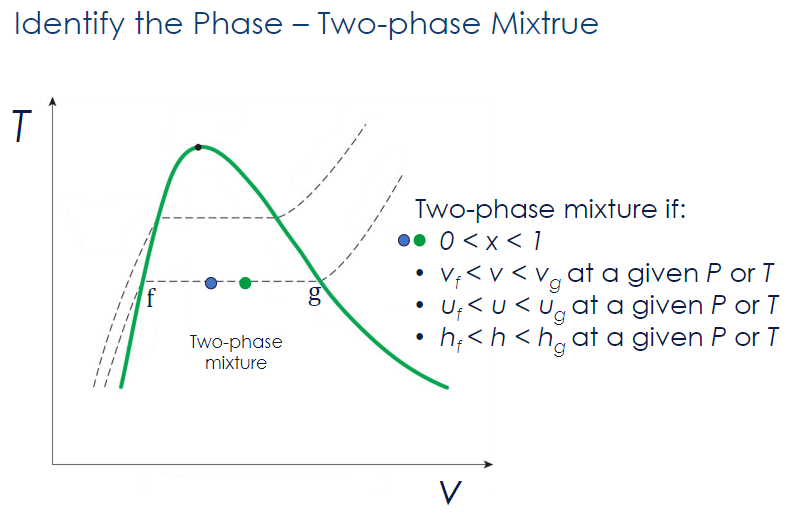
Subcooled Liquid
A substance is a subcooled liquid if:
- at a given
- at a given
- at a given or
- at a given or
- at a given or
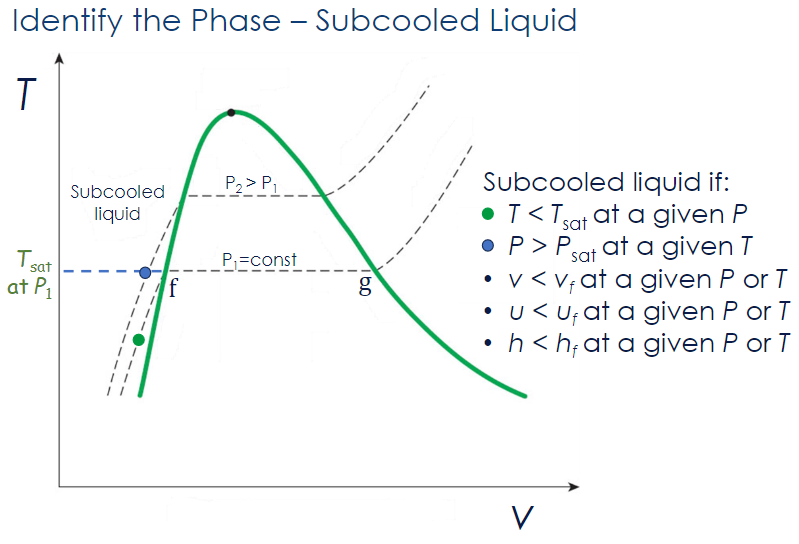
Superheated Vapor
A substance is a superheated vapor if:
- at a given
- at a given
- at a given or
- at a given or
- at a given or
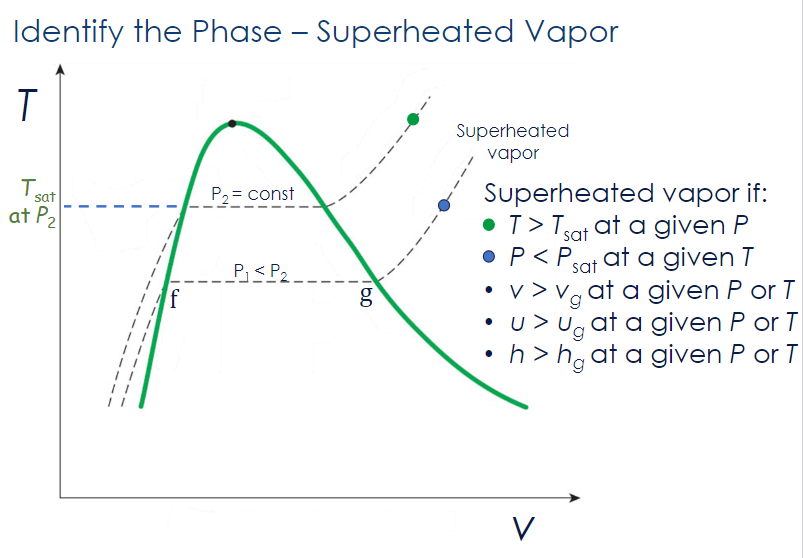
Two-Phase Mixture
A substance is a two-phase mixture:
- at a given or
- at a given or
- at a given or