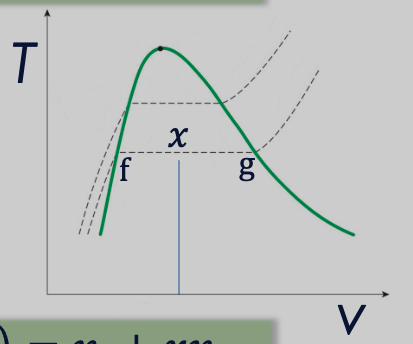
Quality is a thermodynamic property that defines the state of a two-phase mixture. It’s also called a dryness fraction.
- Saturated liquid has
- Saturated vapor has
We can apply the “lever rule”to calculate mass-average properties at saturated states (at and ).
- The point corresponds to the saturated liquid state. 1. At this point, the substance is entirely in the liquid phase but is about to start vaporizing. This is also known as the “boiling point” or “liquid saturation point.”
- The usually corresponds to the saturated vapor state. At this point, the substance is entirely in the vapor phase but is about to start condensing. This is also known as the “dew point” or “vapor saturation point.”
Mass-average: Overall properties based on the amount of vapor and of liquid present.