Constant-volume specific heat is a type of specific heat, signifying the energy required to raise the temperature of a unit mass of a substance by one degree at constant volume.
Since the volume is constant, all of the energy transfer goes into changing the temperature, such that we have
Thus, is is given by:
The variation of specific heats with temperature is smooth and may be approximated as linear over small temperature intervals, so our equation above using is valid if is small (a few hundred degrees).
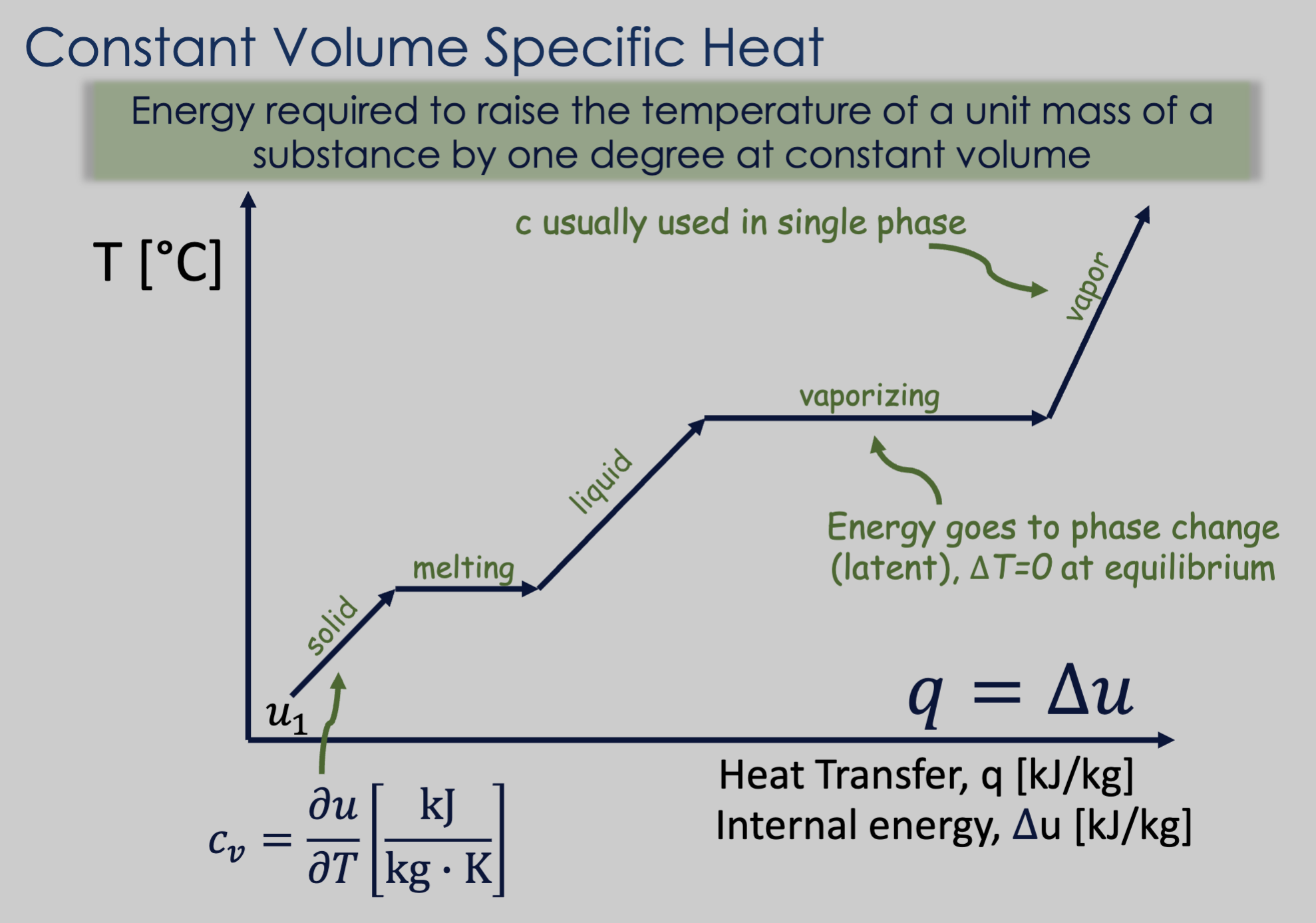
- The flat (constant temperature) parts of the graph are parts where the added energy is going toward breaking toward breaking intermolecular bonds (latent energy), not temperature change (sensible energy).