Per the second law of thermodynamics, real processes contain irreversibilities like heat transfer and friction. Entropy is a thermodynamic property of a system used to quantify the irreversibility of a process or cycle.
Entropy is given as:
- Intrinsic property:
- Extrinsic property:
Specifically, entropy is a measure of the number of possible ways energy can be distributed in a system of molecules.
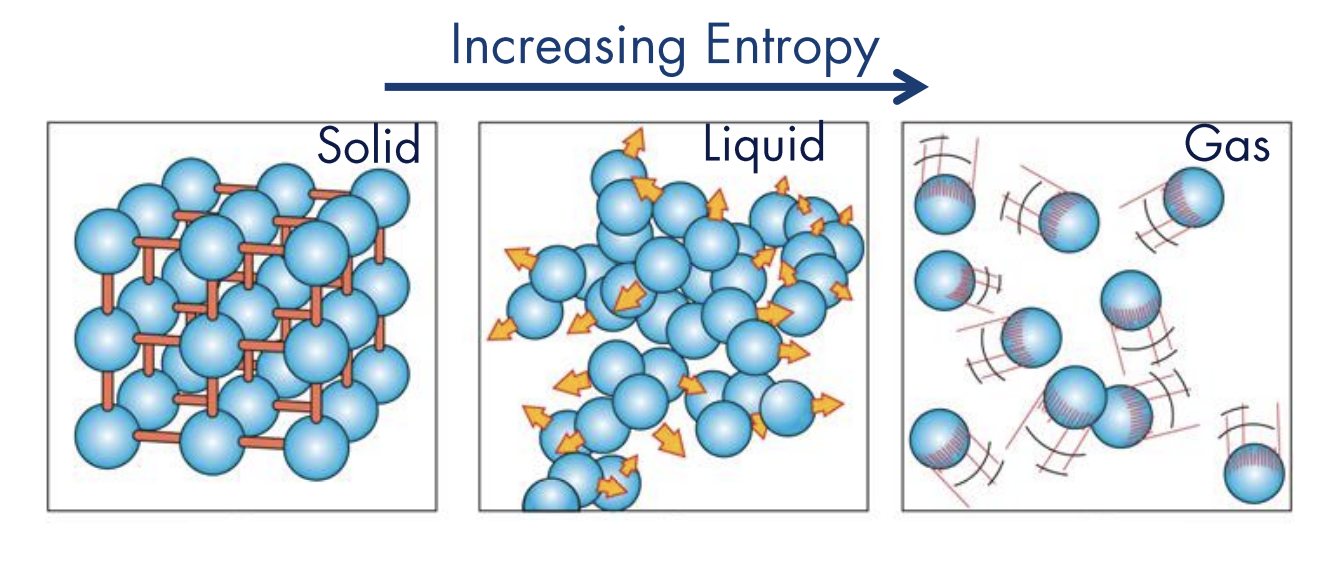
For a perfect crystal of a pure substance, microscopic state is certain (“third law”), such that .
Increase of Entropy Principle
The total entropy (system + surroundings) never decreases. This can be expressed as:
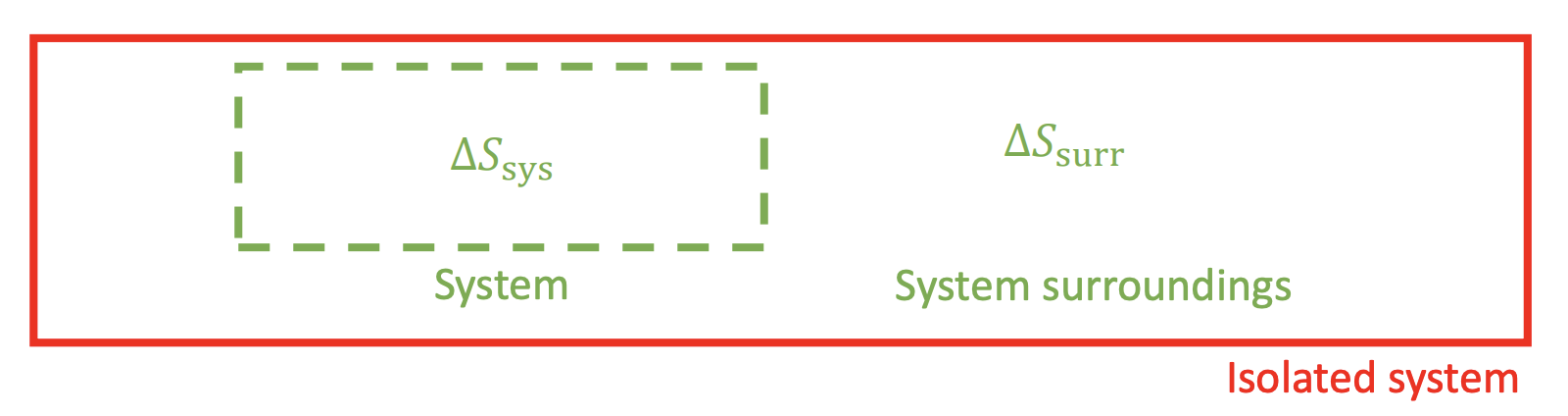
Some cases for :