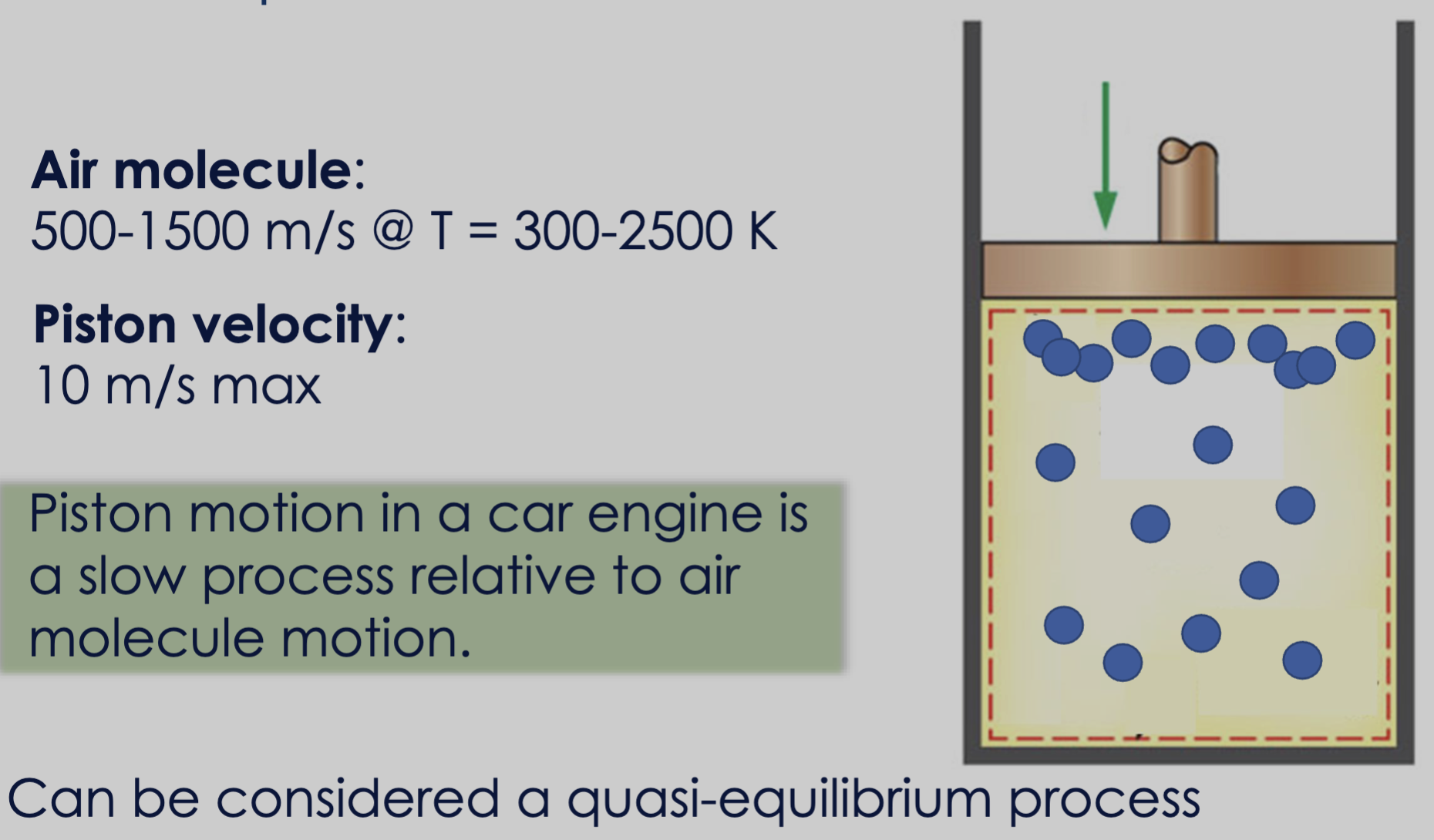
A system will inherently go through intermediate non-equilibrium states during a process.
A quasi-equilibrium process is sufficiently slow, such that the system remains infinitesimally close to thermodynamic equilibrium throughout.
Examples
- Nickel ball that is cooling in such a way that it has uniform temperature distribution.
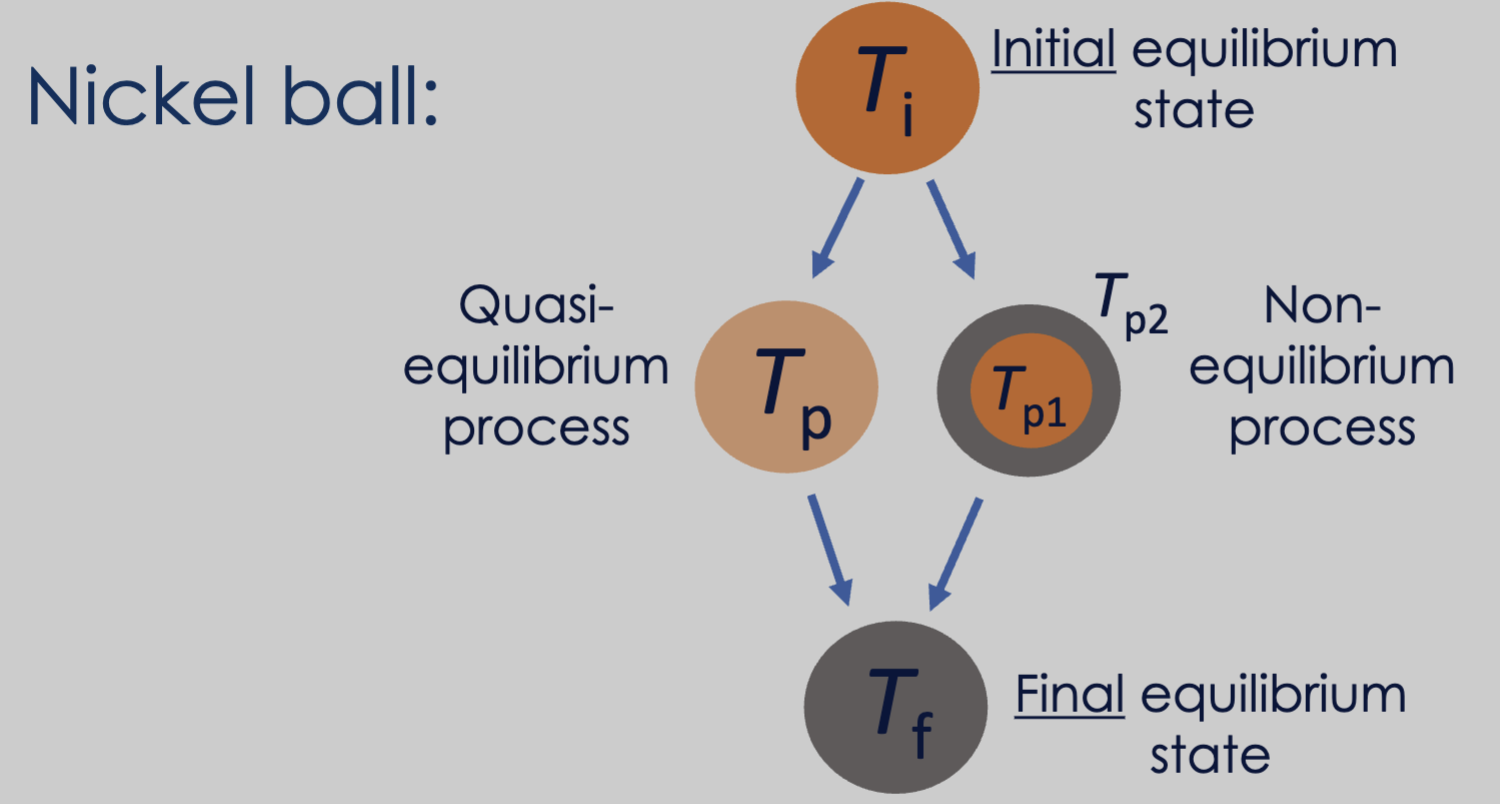
- Piston motion in a car engine is a slow process relative to air molecule motion, such that it can be considered a quasi-equilibrium process.