At the interface between a liquid and a gas, or between two immiscible liquids, forces develop in the liquid surface which cause the surface to behave as if it were a “skin” or “membrane” stretched over the fluid mass. Although such a skin is not actually present, this conceptual analogy allows us to explain several commonly observed phenomena. For example:
- A steel needle or a razor blade will float on water if placed gently on the surface because the tension developed in the hypothetical skin supports it.
- Small droplets of mercury will form into spheres when placed on a smooth surface because the cohesive forces in the surface tend to hold all the molecules together in a compact shape.
- Similarly, discrete bubbles will form in a liquid.

These various types of surface phenomena are due to the unbalanced cohesive forces acting on the liquid molecules at the fluid surface. Molecules in the interior of the fluid mass are surrounded by molecules that are attracted to each other equally. However, molecules along the surface are subjected to a net force toward the interior. The apparent physical consequence of this unbalanced force along the surface are subjected to a net force toward the interior. The apparent physical consequence of this unbalanced force along the surface is to create the hypothetical skin or membrane.
A tensile force may be considered to be acting in the plane of the surface along any line in the surface. The intensity of the molecular attraction per unit length along any line in the surface is called the surface tension and is designated by the Greek symbol with units of
For a given liquid the surface tension depends on temperature as well as the other fluid it is in contact with at the interface.
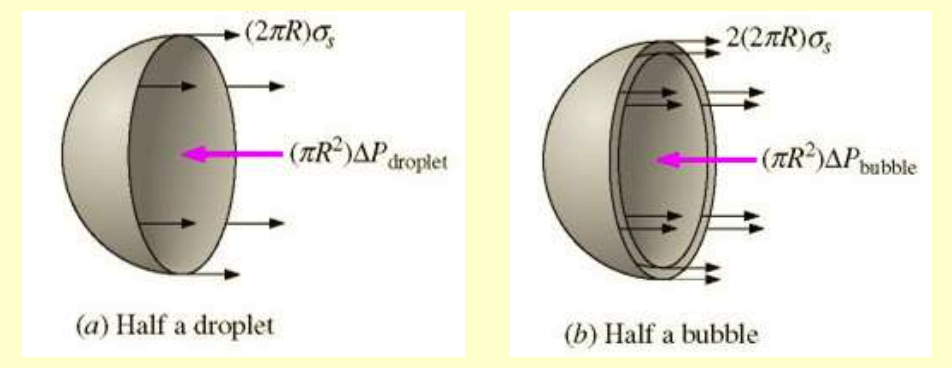
If a spherical drop is cut in half, the drop developed around the edge due to the surface tension must be balanced by the pressure difference, , between the internal pressure, , and the external pressure, , acting over the circular area,
Thus, for a droplet or bubble, we have:
or
such that .