A common phenomena associated with surface tension is the rise and fall of a liquid in a capillary tube. If a small open tube is inserted into water, the water level in the tube will rise above the water level outside the tube. In this situation, we have a liquid-gas-solid interface.
For the case illustrated, there is an attraction (adhesion) between the wall of the tube and liquid molecules, which is strong enough to overcome the mutual attraction (cohesion) of the molecules and pull them up the wall. Hence, the liquid is said the wet the solid surface.
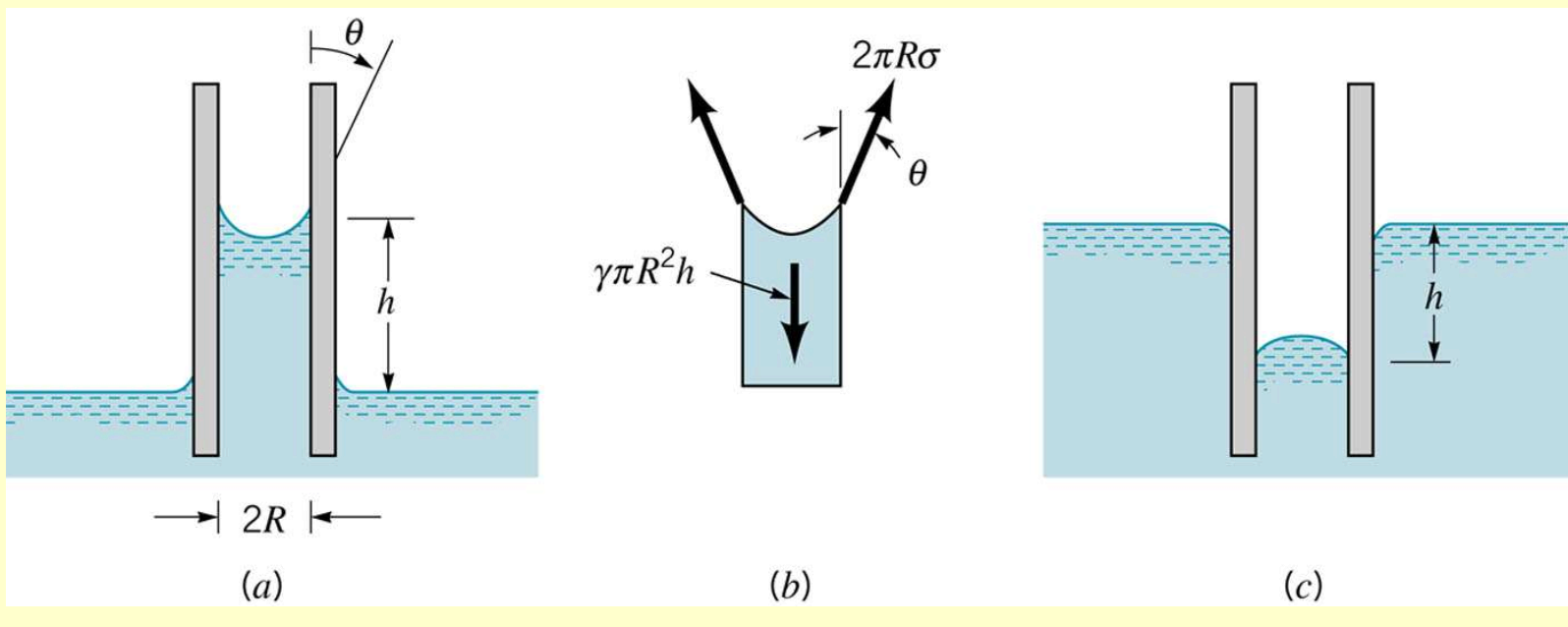
The height, , is governed by the value of the surface tension, , the tube radius, , the specific weight of the liquid, , and the angle of contact, , between the fluid and the tube. From the free-body diagram above, we see that the vertical force due to surface tension is equal to and the weight is and these two forces must balance for equilibrium. Thus,
so that the height is given by the relationship
The angle of contact is a function of both the liquid and the surface. It is clear from above that the height is inversely proportional to the tube radius, and therefore, as indicated by the figure in the margin, the rise of a liquid in a tube as a result of capillary action becomes increasingly pronounced as the tube radius is decreased.